How to Make a Battery That Lasts (Practically) Forever
by Joshua
Krause
 When going off the
grid, it’s safe to assume that most folks will be relying on solar panels for
much of their electrical needs. However, a lack of sunlight can present a few problems
for any would-be prepper. If only there was a cheap and simple way to
supplement a solar panel array on those cloudy days.
When going off the
grid, it’s safe to assume that most folks will be relying on solar panels for
much of their electrical needs. However, a lack of sunlight can present a few problems
for any would-be prepper. If only there was a cheap and simple way to
supplement a solar panel array on those cloudy days.Fortunately, there is such a way, and I’m willing to bet that most of you reading this have never heard of it (I hadn’t until recently). It’s called a Dickens Magnesium Battery after it’s inventor, Stephen Dickens; though the principles behind its function have been around for a very long time. If anything it may be more of a rediscovery, than a completely novel idea.
This device is also called a “Galvanic Cell,” which has been around since the late 1700s, and possibly even earlier if the theories surrounding the Baghdad Battery are to be believed. It generates small electrical currents by capturing the energy produced by the corrosion of a metal.
In this case, the Dickens battery uses the magnesium as its source of electricity, which many of you probably already know if you’ve ever used a fire starter, is a very energy dense material. The design is simple enough that pretty much anyone can make it.
You start out with thick, magnesium rods, which you can buy on Ebay. After that, you’ll need to fasten a metal electrode to the rod with a hose clamp. The metal used for this step is never specified, so feel free to try out a few different metals to see what nets you the best results (more on that in a moment).
After that, you wrap the rod in porous foam, and then coil copper wire around the foam. The idea is to allow water to pass through the foam, but to keep the copper from touching the electrode. Doing so won’t cause anything catastrophic, but your battery will stop producing energy.
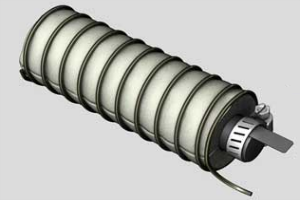
After it’s all said and done, it should look like this:
But what is it capable of?
Each cell should produce about 1.5 volts, and anywhere from 20mah to 100mah. You’ll notice that the current has a fairly wide range. That’s because this invention hasn’t been around very long, and it’s hard to say what will allow it operate at its optimum efficiency. That’s what I was talking about before with the metal electrode. You’ll have to try a few different metals to see what works best.
Although it doesn’t produce a whole lot of energy, it is pretty cheap, and it will last a really long time. Depending on the current you get from it, it may last more than a year. Maybe even longer. It’s hard to say because to my knowledge, nobody has ever completely depleted the magnesium.
And with 1.5 volts, you can connect 8 of these to produce 12 volts of direct current. Coincidentally, that is exactly what you need if you want to connect it to a deep cycle battery, which are typically used to store the energy produced by solar panels. If you manage to get 8 of these producing 100mah of current, you’ll be pumping a steady stream of 1.2 watts of energy, 24 hours a day, for at least 9 months.
At that point, you’ll have to take the battery apart, and scrape the corrosion buildup off the magnesium and the copper wire. And that’s pretty much the only maintenance you’ll have to do. It’s not a lot of energy, but it adds up after a while, and it’ll be able to supplement a small portion of your energy needs when the sun isn’t out. Or if you don’t mind rapidly depleting your magnesium, you can also add salt to the tap water, which will produce more energy.
For a more detailed description of this device, check out the full instructions [below] for its construction, and hopefully you’ll soon be enjoying your new magnesium battery bank.
From Ready Nutrition
@ http://readynutrition.com/resources/how-to-make-a-battery-that-lasts-practically-forever_21062015/
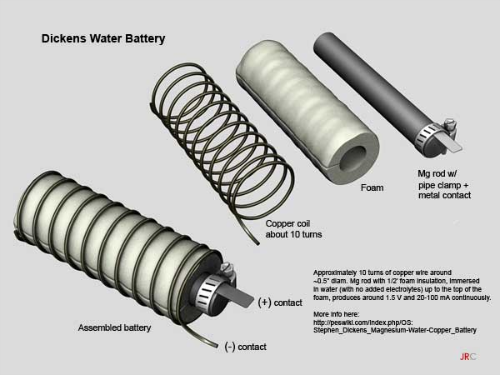
OS: Stephen Dickens Magnesium-Water-Copper Battery

A
$3 magnesium rod surrounded by (not touching) a copper coil in water, produces
1.5 V and enough current to power a wall clock.
Would you like to be able to build an AA battery for emergency preparedness, or just for self-reliability? Consider Stephen Dickens' Water Battery/Generator using Magnesium and Copper Electrodes with Foam Insulator.
It's a lot bigger than an AA, but is cheap and easy to build.
Variations of the concept have been around for more than a century. This one, though probably not unique, appears to be noteworthy given its simplicity. A kindergartener could build this and afford to buy the parts needed.
Briefly, it entails approximately 10 turns of 10-12-gauge copper wire loosely over foam around a Magnesium rod of dimensions: ~0.5" diameter by 3.5" long. The rod is loosely wrapped by 1/2-inch foam insulation. An electrode is attached to the top of the Mg rod with a hose clamp. The assembly is immersed in water (with no added electrolyte) up to the top of the foam.
Adding electrolyte, while increasing amperage, diminishes the life of the Mg. Without electrolyte, the battery can last longer. It produces around 1.5 V and a few mA continuously. Every 9 months or so, the magnesium rod and copper wire should be briefly sanded to remove build-up. The Dickens Battery uses no salts, acids, or added chemicals.
Other people have built Mg-Cu-H2O cells and posted them to YouTube. What makes this unusual is that Stephen doesn't use electrolyte, and this enables the Mg to have longevity that makes the battery practical.
Apparently the power output is a function of the Mg depletion. The question is whether the addition of electrolytes merely speeds up the Mg depletion (resulting in increased power), or if the electrolytes themselves speed up the Mg depletion.
The objective here is to characterize the phenomena, optimize the output and efficiency, miniaturize it, identify alternate variations using other materials that might work even better, and facilitate its dissemination as a solution, if it can be made feasible.
Who can come up with the most cost-effective, easy-to build battery design, using materials that are readily available?
Anyone is welcome to participate in this contest. But if you make money based on this concept presented by Stephen Dickens, we ask that you share a royalty.
Non-exclusive license
terms
Stephen
has agreed to let NEST help him open source this technology, this page being the
home page for that project. Since he has graciously chosen to share his
technology with the world in this manner, rather than going through the
traditional route of confidentiality, patents, etc... (though whether a patent
could be awarded is unlikely), we request that you likewise honor him and do
the right thing by remitting a 5% royalty for any commercial applications of
this technology. Royalties should be remitted to NEST, who has an agreement
with Stephen to share 60% with him, while retaining 40% to finance the
administration and propagation of this project.
Please direct your royalty payments to:
New Energy Systems Trust
c/o Chip Paul, Treasurer
9717 E 42nd Street
Tulsa, OK 74146 USA
email: chip.p@energynest.org
phone: +1-918.289.0000
Note: NEST is not taking donations for Steve. You can donate to his project via his website, listed below.
Cautions
Magnesium is highly flammable. People will whittle shavings of Mg from a rod to use for fire starter. If a Mg rod catches fire (e.g. from a flame source, or even just from grinding), it cannot be doused by submerging in water but will continue to burn very hot until the Mg is consumed. That's why they use Mg rods for under-water welding. You can possibly douse it by covering it in sand, but it can still re-ignite.
As for health or pollution, Mg is not an issue. It is found in food. It is non-polluting.
Official Websites
- This PESWiki page is the official page for this open source project.
- blinkyblue {dot} zapto {dot} org - Steve's original site (The zapto domain is considered a "spam" site by Mediawiki, so we can't link directly to it.)
- My Inventions Calendar By Stephen Dickens
- http://www.youtube.com/user/stevensrd1 - Steve's YouTube Channel
Interviews
- Sterling Interviews Steve (FreeEnergyNow; June 2, 2012)
Videos
Instructions
Parts List

5 Pack by GalliumSource.
- Magnesium rod of dimensions: ~0.5" diameter by 3.5" long.
- US Sources:
- We recommend http://GalliumSource.com > Magnesium Metal > 5 Pack for $12.99
- Ebay by GalliumSource
- Bare copper wire (e.g. 12- or 14-gauge household wiring ground wire), about 1 foot long.
- Tap water
- Small container to hold water, bigger than 6 oz.
- A way to cap the container to prevent evaporation of the water. The lid will need to be penetrated with wires to connect to the electrodes.
- Silicon or other sealant to put around the holes in the lid (for the two protruding wires) to make an air-tight connection to prevent evaporation.
- Alligator clips and wires to run from the electrodes to what is being powered.
- Sand (enough on hand to completely cover the Mg. rod in case it should catch fire; in order to douse the fire.
- (Note, no electrolyte is needed or suggested, as it causes corrosion and diminishing of the Mg rod.)
Optional / Supplemental Parts
- Capacitor, to run in parallel with the electrodes, sometimes helps. 450 V, 100 uF; or 10 V 2200 uF, or 200V 220 uF
- Note, this needs to be characterized, optimized, in this open source project.
Tools (optional)
- A multimeter would be good to have to measure volts and amps.
- A caulk gun for dispensing silicon would come in handy if you wish to make an airtight seal around the electrodes to prevent evaporation of the water.
Assembly
1.
Fasten
a short piece of metal to the end of the Mg rod with the hose clamp.
2.
Use
the handle of a screwdriver to wrap around 10 turns of copper wire to then slip
over the foam on the Mg rod. It should fit loosely, not tightly.
3.
The
rod is wrapped by 1/2-inch foam insulation. The assembly is immersed in water
(with no added electrolyte) up to the top of the foam. It produces around 1.5 V
and 20-100 mA continuously. Every 9 months or so, the magnesium rod and copper
wire should be briefly sanded to remove build-up.
Assembly Diagram
Here's a simplified graphic:

Operation Instructions
1.
Assemble
the device per the above instructions.
2.
Use
an alligator clip to connect the positive electrode from the cell to the
positive connector of what it is powering, and the negative electrode to the
negative connector of what it is powering. (The AA battery container usually
has a diagram showing a AA battery, with a "+" on one end and a
"-" on the other.) You will sometimes need to be creative in figuring
out how to make an electrical connection to the target device +/- connectors.
3.
If
there isn't enough juice to run the device, combine two in parallel, or more,
until there is enough juice.
Designer Profile:
Stephen Dickens
 Stephen
Ralph Dickens was born in Lexington, NC, and grew up in Salisbury, NC. Born May
17, 1969, as of June, 2012, he is 43 years old. His mother was the late Daisy
Boone, and his father is Stephen Saddler. His parents split up before Stephen
was born, so he ended up with his sister's father's last name, which is
Dickens.
Stephen
Ralph Dickens was born in Lexington, NC, and grew up in Salisbury, NC. Born May
17, 1969, as of June, 2012, he is 43 years old. His mother was the late Daisy
Boone, and his father is Stephen Saddler. His parents split up before Stephen
was born, so he ended up with his sister's father's last name, which is
Dickens. Steve's late sister was Ann Ghent. He lives with his wife, Sandra and his daughter Krystal and son, Wayne.
Steve quit school at an early age, so he is self taught in most everything. He has had an interest and has been fiddling with electronics, science and inventing things since he was kid.
Replications…
Continues at source: From PESWicki @ http://peswiki.com/index.php/OS:Stephen_Dickens_Magnesium-Water-Copper_Battery
For more information about clean (and/or free) energy
systems see http://nexusilluminati.blogspot.com/search/label/clean%20electricity
- Scroll down
through ‘Older Posts’ at the end of each section
Hope you like this
not for profit site -
It takes hours of work every day by
a genuinely incapacitated invalid to maintain, write, edit, research,
illustrate and publish this website from a tiny cabin in a remote forest
Like what we do? Please give anything
you can -
Contribute any amount and receive at
least one New Illuminati eBook!
(You can use a card
securely if you don’t use Paypal)
Please click below -
Spare Bitcoin
change?
For further enlightening
information enter a word or phrase into the random synchronistic search box @
the top left of http://nexusilluminati.blogspot.com
And see
New Illuminati – http://nexusilluminati.blogspot.com
New Illuminati on Facebook - https://www.facebook.com/the.new.illuminati
New Illuminati Youtube Channel - http://www.youtube.com/user/newilluminati
New Illuminati on Google+ @ For
New Illuminati posts - https://plus.google.com/u/0/+RamAyana0/posts
New Illuminati on Twitter @ www.twitter.com/new_illuminati
New Illuminations –Art(icles) by
R. Ayana @ http://newilluminations.blogspot.com
The Her(m)etic Hermit - http://hermetic.blog.com
DISGRUNTLED SITE ADMINS PLEASE NOTE –
We provide a live link to your original material on your site (and
links via social networking services) - which raises your ranking on search
engines and helps spread your info further!
This site is published under Creative Commons (Attribution) CopyRIGHT
(unless an individual article or other item is declared otherwise by the copyright
holder). Reproduction for non-profit use is permitted
& encouraged - if you give attribution to the work & author and include
all links in the original (along with this or a similar notice).
Feel free to make non-commercial hard (printed) or software copies or
mirror sites - you never know how long something will stay glued to the web –
but remember attribution!
If you like what you see, please send a donation (no amount is too
small or too large) or leave a comment – and thanks for reading this far…
Live long and prosper! Together we can create the best of all possible
worlds…
From the New Illuminati – http://nexusilluminati.blogspot.com
No comments:
Post a Comment
Add your perspective to the conscious collective