The Ultimate Medical Breakthrough
Telomerase and Longevity
by Gary Vey
The results of an experiment that offers a glimpse into the future of the human race -- a future when there is no more "getting old" and, most significantly, no cancer - were recently published. But to fully appreciate what has happened, you will need to get the background story first. It is a natural desire to want to live a long life. Despite the claims of eating organic food, vegetarianism or vitamins, the only thing scientifically linked to longevity (besides starvation) is genetics. If your parents and grandparents lived a long life, you will likely live long.
We now understand exactly what causes aging and death -- on the molecular level. Scientists have spent the last decade trying to manipulate the enzymes and protiens that determine how many times our cells can make copies of themselves -- and how long we can live. But they have always faced a dilema: the same conditions that makes a cell live longer are also present in cancer.
About a decade ago, it was discovered that the ends of the DNA molecular strands had a long series of meaningless code ("TTAGGG" in the language of DNA enzymes) that kept repeating and repeating. It was noticed that these strands, called telomeres, were longer in young cells (about 10,000 base pairs) and shorter in older cells (about 5,000 base pairs). When the telomeres got too short, the cell died.
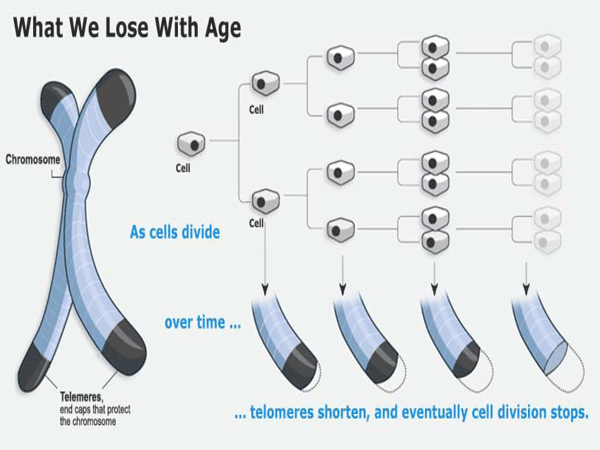
Telomeres are actually chains of molecules. They have often been compared to the blank leaders on film and recording tape which are intended to provide a "buffer", protecting the vital information during handling. Telomeres perform a similar function during the cell replication process.
When a cell divides, the spiral DNA molecule must split in half and reassemble a copy of itself. Protecting the vital DNA molecule from being copied out of synch, telomeres provide a kind of safety zone where mis-alignments (which are inevitable) will not result in any of the important DNA code being lost.
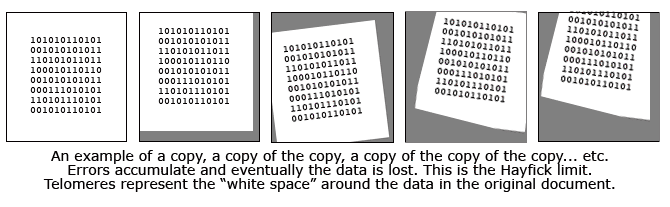
Perhaps the best analogy I have heard is to compare the telomeres to the white margin surrounding an important type written document. In this analogy, the printed text is the vital DNA code while the white space is the "blank" telomeres. Imagine that this paper is repeatedly slapped on a copy machine, a copy is made, and then that copy is used to make another copy. Each time the paper is subject to errors of alignment and these errors accumulate. After enough copying, it is probable that the white space will diminish and some of the actual text will not be copied. That's what happens inside our cells and it is the reason we get old and die.
The Holy Grail (enzyme): Telomerase
The next big discovery was an enzyme called telomerase which was present in cells which regrew their telomeres and was absent in cells that didn't. With their genes for making the enzyme telomerase turned "on", cells were able to repair and regrow telomeres.
As might be expected, the fetus enjoys abundant telomerase. The surprise is that most of the telomerase making genes are turned "off" after birth. Human cells have their longest telomeres at birth, diminishing with each copy they produce and providing a finite number of potential replcations. (the Hayflick limit)
There are some specialized cells that continue to produce telomerase after birth. Tissue like the lining of your mouth and your digestive and immune system need to make many copies to repair the daily natural assault from the environment. But there is another kind of cell that manages to turn "on" the telomerase production, so much so that it becomes virtually immortal -- cancer.
The Cancer Problem
Usually, when a cell makes an error in copying itself, the error will prevent the cell from duplicating itself and it dies before making any copies of the error. So the mistake is limited. But with cancer, cells make errors in copying the DNA code and then, somehow, turn "on" the production of telomerase -- allowing them to make unlimited copies of themselves -- making them immortal. The aberrant cells frequently lose their former function, then rapidly reproduce and outlive normal cells. This is the process that creates tumors.
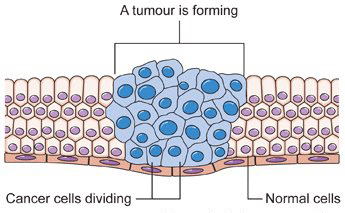
When mice with inactive telomerase (short telomeres) were bred with cancer prone mice (long telomeres), the resulting offspring initially had long telomeres and were highly susceptible to cancer at a young age [3]. But when these offspring were bred for five generations their telomeres became gradually very short and they were free of cancer. The link between telomere length and cancer has since been replicated many times and is widely accepted.
In the past few years, pharmaceutical companies like Geron Corporation and Merck have been conducting clinical trials on vaccines designed to teach the body's immune system to attack cancerous cells producing telomerase [*]. Telomerase seems always to be viewed as a negative enzyme. Part of the problem is that cancer is considered a disease, where death is not. But that may soon change.
The early belief was that if humans could activate or turn "on" their telomerase, their cells could potentially be immortal BUT they would likely die from the cancer that is associated with longer telomeres. This was contradicted by some experiments with mice, bred to manipulate their telomerase activity.
Harvard scientists reverse the ageing process in mice. Harvard scientists claim to be a step closer to reversing the ageing process after rejuvenating worn out organs in elderly mice. The experimental treatment developed by researchers at the Dana-Farber Cancer Institute, Harvard Medical School, turned weak and feeble old mice into healthy animals by regenerating their aged bodies [1]. "What we saw in these animals was not a slowing down or stabilisation of the ageing process. We saw a dramatic reversal -- and that was unexpected." --Ronald DePinho, who led the study, which was published in the journal Nature. At Harvard, they bred genetically manipulated mice that lacked an enzyme called telomerase that stops telomeres getting shorter. Without the enzyme, the mice aged prematurely and suffered ailments, including a poor sense of smell, smaller brain size, infertility and damaged intestines and spleens. But when DePinho gave the mice injections to reactivate the enzyme, it repaired the damaged tissues and reversed the signs of ageing. "These were severely aged animals, but after a month of treatment they showed a substantial restoration, including the growth of new neurons in their brains."--DePinho. Lynne Cox, a biochemist at Oxford University, said the study was "extremely important" and "provides proof of principle that short-term treatment to restore telomerase in adults already showing age-related tissue degeneration can rejuvenate aged tissues and restore physiological function." DePinho said none of Harvard's mice developed cancer after the treatment. The team is now investigating whether it extends the lifespan of mice or enables them to live healthier lives into old age. |
And now the new research...
OK, let's talk. You and I both know that the future of the human race is headed in the direction of longer and healthier lifespans. The telomere experiments have thus far involved breeding mice to have shorter telomeres. Applying this to human life-extension would require selective breeding of humans in a carefully controlled program, over many generations. But it could be done.
Until now, there has been no way to switch "on" the telomerase quickly. Until now the human race was destined to be artificially evolved, creating a special "family" of telomerase enhanced beings that would intermarry and enjoy a long and disease free life.
But some scientists in Barcelona have just published their successful results of turning "on" telomerase in mice with a single treatment given to ordinary, healthy individuals of both adult and aged groups. This was achieved through a novel approach in gene therapy. (Published in the journal EMBO Molecular Medicine)
The gene therapy consisted of treating the animals with a DNA-modified virus, the viral genes having been replaced by those of the telomerase enzyme. Once the animal is infected, the virus acts as a vehicle depositing the telomerase gene in the cells, which then begin to manufacture it. Once the telomerase was being produced, the scientists noticed that the treated mice had lengthened their telomeres and appeared rejuvenated.
The experiments suggest that there is an ideal time when the treatment is most effective. They tested mice that were one year old (considered adult) and two years old (considered old mice). Mice treated at the age of one lived longer by 24% on average, and those treated at the age of two, by 13%. The therapy, furthermore, produced an appreciable improvement in the animals' health, delaying the onset of age-related diseases -- like osteoporosis and insulin resistance -- and achieving improved readings on aging indicators like neuromuscular coordination. These findings were similar to the Harvard study (described above) but did not involve selective breeding.
But the most surprising discovery was that, once again, the mice were free from cancer!
Scientists speculate that, if a healthy cell receives telomerase, it becomes less likely to make copying errors and better able to fight against disease. The telomerase benefits the entire body's systems, including our immunity and ability to rejuvenate.
Future Humans
In 2007, Blasco's group demonstrated that it was feasible to prolong the lives of transgenic mice, whose genome had been permanently altered at the embryonic stage, by causing their cells to express telomerase and, also, extra copies of cancer-resistant genes. These animals live 40% longer than is normal and also do not develop cancer.
Messing with a human embryo is something no moral scientists are prepared to do at present. The new research provides a way of achieving a longer and healthier life without conflicts with morality or religion.
It is believed now that when the telomerase production is switched "on" at an early adult age (as this new study shows), certain anti-cancer genes are also activated. Also, when the cells are younger, there is less likelihood that there are large numbers of aberrant cells that could be made immortal by the treatment.
Also important is the kind of virus employed to carry the telomerase gene to the cells. The researchers selected demonstrably safe viruses that have been successfully used in gene therapy treatment of hemophilia and eye disease. Specifically, they are non-replicating viruses derived from others that are non-pathogenic in humans.
This study is viewed primarily as a proof-of-principle that telomerase gene therapy is a feasible and generally safe approach to improve healthspan and treat disorders associated with short telomeres.
"Aging is not currently regarded as a disease, but researchers tend increasingly to view it as the common origin of conditions like insulin resistance or cardiovascular disease, whose incidence rises with age. In treating cell aging, we could prevent these diseases." [2]
We don't know yet whether the virus treatment can transmit this turned "on" telomerase to offspring. Probably not. Female eggs are made in the fetus before the mother's birth and would presumably be isolated from the treatment. Male sperm, on the other hand, are made-to-order and could possibly carry the genes. Right now it's looking more like an upgrade rather than whole new race.
There have been some data showing that colo-rectal cancer could result from extra long telomeres (or extra short ones) which suggests that there is an optimal range of telomere length that is between immortal and cancerous. So some maintenance to modulate telomere length might be required in a long lived population. [4]
There are so many questions to be answered, and yet to be asked. What is the perfect age for the treatment? How much will it cost? Will it be universally available or restricted... by whom? Regardless, telomerase therapy is coming to humanity very soon.
While we're waiting, could telomere length be used by health or life insurance companies to determine rates? This video [below] addresses some of these issues.
Perhaps in the not too distant future, we will discover that side effects of the treatment are loss of hair, gray skin and enlarged eyes and, having discovered how to travel back in time, we will examine and take samples of our previous ancestors who suffered from a disease called death.
* * *
Notes:
Virginie Ribaud, Cyril Ribeyre, Pascal Damay, David Shore. DNA-end capping by the budding yeast transcription factor and subtelomeric binding protein Tbf1. The EMBO Journal, 2011; DOI: 10.1038/emboj.2011.349
Franck Gallardo, Nancy Laterreur, Emilio Cusanelli, Faissal Ouenzar, Emmanuelle Querido, Raymund J Wellinger and Pascal Chartrand. Live cell imaging of telomerase RNA dynamics reveals cell cycle-dependent clustering of telomerase at elongating telomeres. Molecular Cell, Volume 44, Issue 5, 819-827, 9 December 2011 DOI: 10.1016/j.molcel.2011.09.020
Bettina A Moser, Ya-Ting Chang, Jorgena Kosti, Toru M Nakamura. Tel1ATM and Rad3ATR kinases promote Ccq1-Est1 interaction to maintain telomeres in fission yeast. Nature Structural & Molecular Biology, 2011; DOI: 10.1038/nsmb.2187
Stanford University Medical Center (2008, March 20). Researchers Unmask Proteins In Telomerase, A Substance That Enables Cancer. ScienceDaily. Retrieved May 17, 2012, from http://www.sciencedaily.com/releases/2008/03/080320120834.htm
[2] Bruno Bernardes de Jesus, Elsa Vera, Kerstin Schneeberger, Agueda M Tejera, Eduard Ayuso, Fatima Bosch, Maria A. Blasco. Telomerase gene therapy in adult and old mice delays ageing and increases longevity without increasing cancer. EMBO Molecular Medicine, 2012 (in press) DOI: 10.1002/emmm.201200245
[4] American Association for Cancer Research (2010, October 28). Telomere length affects colorectal cancer risk. ScienceDaily. Retrieved May 20, 2012, from http://www.sciencedaily.com/releases/2010/10/101028132257.htm
From Viewzone @ http://www.viewzone.com/telomerase.html
For more information about longevity see http://nexusilluminati.blogspot.com/search/label/immortalism
Help This Unique Independent Site to Inform You
Donate any amount and receive at least one New Illuminati eBook!
Just click in the jar -
For further enlightening information enter a word or phrase into the search box @ New Illuminati or click on any label/tag at the bottom of the page @ http://nexusilluminati.blogspot.com
And see
New Illuminati – http://nexusilluminati.blogspot.com
New Illuminati on Facebook - https://www.facebook.com/the.new.illuminati
New Illuminati Youtube Channel - http://www.youtube.com/user/newilluminati/feed
The Her(m)etic Hermit - http://hermetic.blog.com
The Prince of Centraxis - http://centraxis.blogspot.com (Be Aware! This link leads to implicate & xplicit concepts & images!)
This site is published under Creative Commons Fair Use Copyright (unless an individual item is declared otherwise by copyright holder) – reproduction for non-profit use is permitted & encouraged, if you give attribution to the work & author - and please include a (preferably active) link to the original along with this notice. Feel free to make non-commercial hard (printed) or software copies or mirror sites - you never know how long something will stay glued to the web – but remember attribution! If you like what you see, please send a small but heartfelt donation or leave a comment – and thanks for reading this far…
Live long and prosper!
From the New Illuminati – http://nexusilluminati.blogspot.com
No comments:
Post a Comment
Add your perspective to the conscious collective